[ad_1]
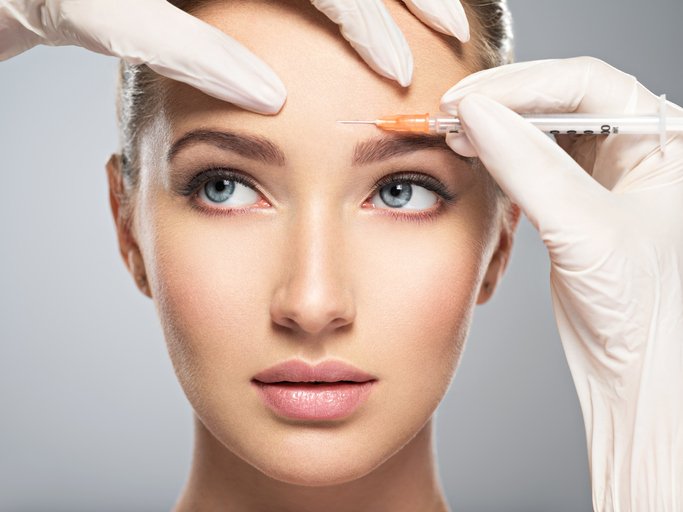
A novel botulinum toxin type A that could potentially compete with Botox, DaxibotulinumtoxinA for Injection (DAXI) is currently under Biologics License Application (BLA) review in the US.
Under this agreement, Aji Bio-Pharma will serve as a dual supply source and provide drug product manufacturing services for Revance at the company’s aseptic manufacturing facility in San Diego, California.
The deal will serve to bolster Revance’s supply chain resiliency, said Brian Blagg, vice president, engineering & supply chain at that next-generation neuromodulator product developer.
Phase 3 trials
In February this year, the US Food and Drug Administration (FDA) accepted, for review, Revance Therapeutic’s BLA for DAXI in the treatment of moderate to severe glabellar – frown – lines, based on the results of Phase 3 trials.
Those Phase 3 studies demonstrated that half of the patients treated with DAXI maintained none or only mild frown lines for at least 24 weeks (around 6 months), after a single treatment. Additionally, frown lines did not return to their pre-treatment severity for at least 26–28 weeks for half of the patients treated, said the Silicon Valley-based biotechnology company.
DAXI is also being evaluated for the full upper face, including glabellar lines, forehead lines and crow’s feet.
Demand for botulinum type A procedures has soared 845% since 2000, according the 2018 National Plastic Surgery Statistics by the American Society of Plastic Surgeons (ASPS).
Along with the aesthetic side push, Revance is assessing DAXI in two therapeutic indications – cervical dystonia and adult upper limb spasticity. The drug received an FDA orphan drug designation in 2017 for the treatment of cervical dystonia.
Developing a Botox copy
And, in parallel to the development of DAXI, the company has partnered with Viatris, formerly Mylan, to develop a biosimilar to Botox.
Last year, Revance said that developing a Botox biosimilar was “challenging, but not impossible” in reply to AbbVie’s rejection of the possibility of a copy of the blockbuster coming to market.
In June 2019, on acquiring Botox maker, Allergan, Richard Gonzalez, CEO of AbbVie, said, “For a variety of technical reasons, I would tell you that it’s highly unlikely that we would see a biosimilar against Botox for a long, long time, if ever.”
Gonzalez’s comments were put to Revance CEO, Daniel Browne, when his company presented its second-quarter results last year. He took a different position to his counterpart at AbbVie, outlining how Revance can clear the barriers to developing a Botox biosimilar based on his company’s extensive experience working on botulinum toxins and the investments it had made in R&D, both in terms of assets and analytical capabilities.
[ad_2]
Source link